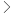| Module 1 |
- Atomic structure:
- Extranuclear structure, line spectrum of hydrogen atom; quantization of energy (Planck’s equation = HV), Bohr model of the atom and its limitations, Sommerfeld’s modification (elementary idea), the four quantum numbers, ground state electronic configurations of many-electron atoms and monatomic ions, the Aufbau principle, Pauli’s exclusion principle and Hund’s Rule, Dual nature of the electron, the concept of atomic orbitals, shapes of S, P and d-orbitals
- Radioactivity and Nuclear Chemistry
- The Periodic table and Chemical families
|
| Module 2 |
- Chemical Bonding and Molecular Structure
- Directionality of covalent bonds, shapes of polyatomic molecules (examples). Concept of hybridization of atomic orbitals involving s.p and d orbitals. Molecular Orbital energy diagrams for homonuclear diatomic species - bond order and magnetic properties, Valence shell electron pair repulsion (VSEPR) concept, (elementary idea) - shapes of molecules, the concept of resonance (elementary idea), resonance structure (examples), Elementary idea about electronegativity, bond polarity and dipole moment, Hydrogen bonding and its effect on physical properties (M.P., B.P. and Solubility)
- Double and complex salts, Werner’s Coordination compounds (examples only), coordination number and geometry (example with CN 4 and 6 only), IUPAC nomenclature of mononuclear coordination complexes (examples)
- Chemical Energetics and Chemical Dynamics
- Enthalpy change in chemical reactions, standard enthalpy of formation, Hess’s law and its applications, bond enthalpy, measurement of enthalpy of reactions, The spontaneity combustion reactions, conservation of energy and the first law of thermodynamics, Mathematical form of First law of thermodynamics, Numerical problems. The spontaneity of the process, entropy, the second law of thermodynamics, elementary idea about entropy change ( ? S) and free energy change ( ? G), the significance of the relation;? G = ? H - T ? S (without derivation), example with gaseous reaction, Numerical problems
- Chemical Dynamics
- Order and molecularity of reactions (determination excluded), First-order reaction, specific rate constant,half-life period, numerical problems, examples of the first-order, pseudo-first-order and second-order reactions.
|
| Module 3 |
- Gaseous State
- Chemical Equilibria, Ionic Equilibria and Redox Equilibria: Chemical Equilibria: The law of mass action, dynamic nature of chemical equilibrium, equilibrium constant (K), Le Chatelier’s principle, Equilibrium constants of Gaseous reactions (Kp and Kc) and the relation between them (examples)
- Ionic Equilibria
- Redox Equilibria
- Standard Electrode potentials (E0 ), Nernst equation and its applications, Electrochemical series, the feasibility of a redox reaction, the significance of Gibb’s equation? GO = nF ? EO (without derivation), e.m.f. of galvanic cells (examples), the stoichiometry of redox reactions, redox titration’s (examples), Numerical problems
|
| Module 4 |
- Atoms, molecules and Chemical Arithmetic
- Chemistry of Solutions
- Colloidal Solution
- Electrolytic Solutions
- Determination of charge of electrons from Faraday’s law, Electrolytic conduction, Conductance, Specific conductance, equivalent conductance and ionic conductance, Molar conductance, Kohlrausch’s law and its application, Numerical Problems.
|
| Module 5 |
- Chemistry of Non-Metallic Elements and their compounds:
- Carbon
- Nitrogen and Phosphorus
- Sulphur and Oxygen
- Halogen Family
- Chemistry in Industry: Large scale production (including Physico-chemical principles where applicable omitting technical details and uses of individual items).
- Heavy Chemicals
- Electro chemicals
- Fuel Gases
- Fertilizer
|
| Module 6 |
A. Chemistry of Metallic elements and their compounds: General principles of metallurgy: Occurrence, the concentration of ores, extraction and purification of metals, minerals wealth of India
- Typical Members
- Principles of chemistry involved in electroplating, anodizing and galvanizing. Metals of Life : Biological roles of Na+, K+, Mg2+, Ca2+ Fe2+, Fe3+, Cu2+, and Zn2+ (elementary idea), myoglobin haemocyanin, chlorophyll (metal ion present and bio function to be mentioned, structure - non evaluative)
- Compounds of metals
|
B. Cement: Composition and setting of Portland cement
| Module 7 |
Chemistry of Organic Compound
Hydrocarbons: Classification of hydrocarbons: Alkane-general methods of preparation and general properties with reactions
- Methane and ethane
- Ethylene
- Acetylene
Aromatic hydrocarbon: o, m, p isomers, Nucleus and side chain, Aromaticity
- Benzene and its homologues
- Toluene and its o, m, p, substituted derivatives, chlorination (hydrolysis of chlorinated products included), side-chain oxidations
Organic Compounds Containing halogens (Haloalkanes and Haloarenes): General method of preparation, properties and reactions, haloform reaction, chloroform and Iodoform, carbylamine reaction, Chlorobenzene, (preparation, properties and uses). Preparation of Grignard reagents and their synthetic applications
|
| Module 8 |
- Organic compounds containing Oxygen
- Alcohols: Methanol and ethanol (from fermentation)
- Ether: Diethyl Ether. Aldehydes and ketones: Formaldehyde, acetaldehyde and acetone
- Carboxylic acids and their derivatives: Formic acids, acetic acid and oxalic acid, acetyl chloride, acetic anhydride, acetamide, Ethyl acetate
- Phenol, Benzaldehyde, benzoic acid, salicylic acid, anthranilic acid, Acidity of carboxylic acid and phenol, the effect of substituents on the acidity of carboxylic acid
|
| Module 9 |
Organic compounds containing nitrogen:
- Methylamine, Ethylamine, Aniline - preparations, properties, reactions and uses.
- Diazonium chloride - preparation, reaction and synthetic application
Polymer: Classification of polymers, natural and synthetic polymers (with stress on their general methods of preparation) and important uses of the following.
- Polythene, Nylon-66, Teflon, PVC, Rubber from natural sources including Vulcanization.
Introduction of Biomolecules: Carbohydrates: Pentoses and Hexoses; Distinctive chemical reaction of glucose, Amino acids: glycine, alanine, aspartic acid, cysteine (structure), Zwitterion structures of amino acids, Peptide bond, ADP and ATP - structure and role in bioenergetics. Nucleic acids - DNA and RNA.
|
| Module 10 |
Environmental Chemistry: Chemical nature of air, water and soil and their role in the environment, common modes of pollution of air, water and soil, the importance of ozone layer, reactions causing ozone layer depletion, Greenhouse effect, smog, pollution of water by domestic and industrial effluents, pollutants - pesticides, fertilizers and plastic.
Application Oriented Chemistry: Main ingredients, their chemical natures (structures not required) and their side effects, if any, of common antiseptics, analgesics, antacids, pain killers, Vitamin C.
- Technical/Domestic/Medicinal uses of Chemicals: Baking powder, Calcium lactate, Boric acid, Borax, Zinc sulphate, oil of wintergreen, Carbolic acid.
Principles of qualitative analysis: Detection of water-soluble non-interfering acid and basic radicals by dry and wet tests from among :
a) Acid Radicals : Cl-, S2-, So2-4, NO-3, Co2-3 b) Basic Radicals : Cu2+, Al3+, Fe2+, Fe3+, Zn2+, Mg2+, Na+, NH 4+. Detection of special elements (N, Cl, Br, I and S) in organic compounds by chemical tests, Identification of functional groups in phenol, aromatic amines, aldehydes, ketones and carboxylic acids.
|