



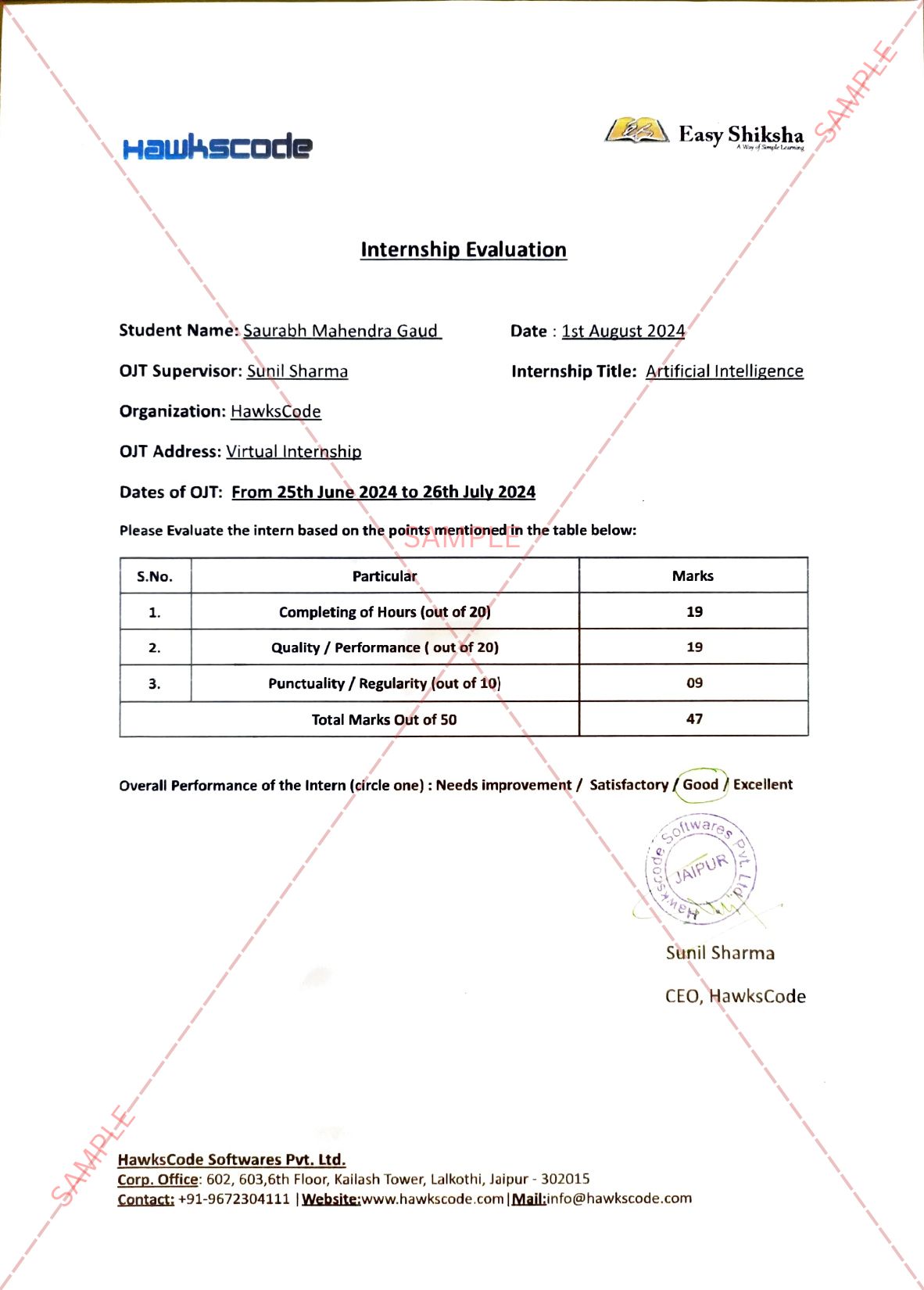




Poster presentation highlighted promising pharmacology and behavioral results on lead candidate, TN-001, in preclinical studies.
TN-001 is under development for both major depressive disorder (MDD) and post-traumatic stress disorder (PTSD).
TN-001 rapidly induces neuroplasticity, including synaptogenesis.
NASSAU, Bahamas, Jan. 14, 2026 /PRNewswire/ -- Transneural Therapeutics, Inc. (Transneural), a neuroscience-focused biotechnology company aiming to transform the treatment of neuropsychiatric and neurodegenerative diseases with novel pharmacotherapies, today presented new data on their lead candidate, TN-001, at the 64th Annual Meeting of the American College of Neuropsychopharmacology (ACNP), taking place January 12-15, 2026 in Nassau, Bahamas.
"Research has shown that neuroplasticity plays a crucial role in the pathophysiology of various neuropsychiatric disorders, including depression and PTSD," said Roger McIntyre, M.D., FRCPC. "With a significant unmet need in both conditions, new treatment approaches are clearly needed. Given this reality, I believe that the neuroplasticity data seen with TN-001 are very encouraging."
"We are excited to present our data on TN-001. The data in our poster provide critical insights for the potential use of TN-001 for the treatment of major depression and other neuropsychiatric conditions. The ability of TN-001 to rapidly induce neurite growth and promote synaptogenesis is particularly encouraging as we continue to explore the potential use of TN-001 for other neuropsychiatric and neurodegenerative conditions," explains Mark A. Demitrack, M.D., Chief Research and Development Officer of Transneural Therapeutics.
Presentation Title: Preclinical Behavioral and Pharmacological Characteristics of TN-001, a Novel, Non-Hallucinogenic Neuroplastogen for the Treatment of Major Depressive Disorder
Key Findings:
About Transneural
Transneural is a preclinical-stage biotechnology company transforming the treatment of neuropsychiatric and neurodegenerative diseases with novel rapid-acting therapies. Transneural is led by globally recognized clinical development and commercialization leaders in neuropsychiatry and neurodegeneration. Transneural's pipeline is focused on G-protein coupled receptors known to be validated drug targets, with therapeutics built on entirely novel chemical scaffolds identified by bridging AI-informed structure/function relationships with applied medicinal chemistry.
About TN-001
Transneural's lead asset, TN-001, is a dual 5-HT2A partial agonist/5-HT2B antagonist. TN-001 is an AI-informed molecule specifically designed to deliver rapid and robust efficacy with a cardiac safety profile that enables daily dosing by the patient at home. TN-001 is in development for MDD and PTSD. In preclinical studies, TN-001 produces rapid neuroplasticity and synaptogenesis, but without the disadvantage of behavioral toxicity.
This press release is not sanctioned by ACNP.
Media Contact
Liz Cogan
media@transneural.com
+1.619.780.7684
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/transneural-therapeutics-presents-preclinical-data-on-lead-candidate-tn-001-at-64th-annual-meeting-of-acnp-2026-302661444.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/transneural-therapeutics-presents-preclinical-data-on-lead-candidate-tn-001-at-64th-annual-meeting-of-acnp-2026-302661444.html
SOURCE Transneural Therapeutics, Inc.


Discover thousands of colleges and courses, enhance skills with online courses and internships, explore career alternatives, and stay updated with the latest educational news..

Gain high-quality, filtered student leads, prominent homepage ads, top search ranking, and a separate website. Let us actively enhance your brand awareness.